WHO announces historic, life-saving malaria vaccine for Africa's children
“A glimmer of hope for the continent”
The World Health Organization (WHO) has recommended the widespread use of a new malaria vaccine across Africa with the potential to save the lives of tens of thousands of children’s lives every year.
“This is a historic moment. The long-awaited malaria vaccine for children is a breakthrough for science, child health and malaria control,” said WHO Director-General Dr Tedros Adhanom Ghebreyesus.
The vaccine is called the RTS,S/AS01 (RTS,S) and WHO recommends it for children in sub-Saharan Africa and other regions with moderate to high malaria transmission.

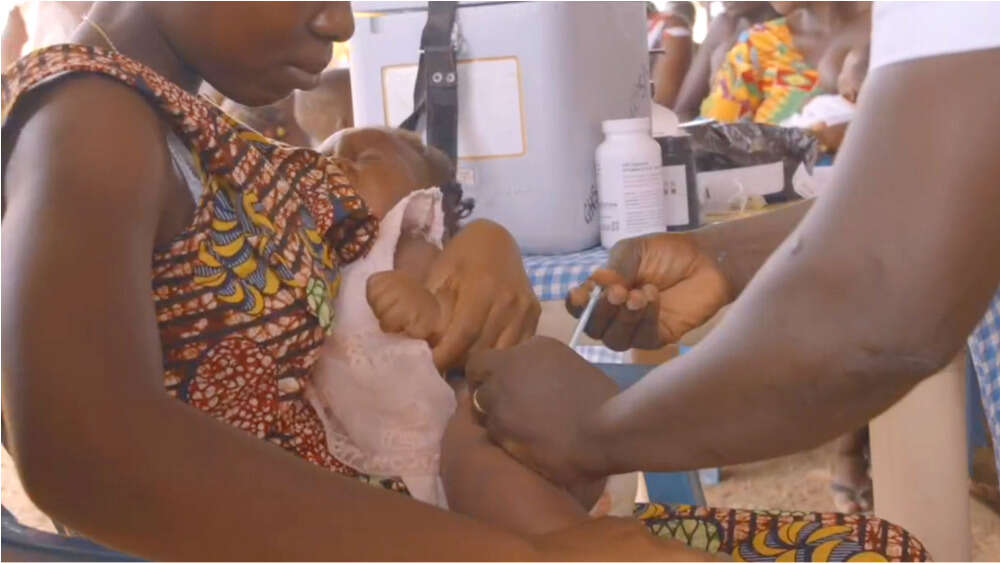
Mothers are briefed ahead of their children’s vaccinations.
Malaria remains a primary cause of childhood illness and death in sub-Saharan Africa. More than 260 000 African children under the age of five die from malaria annually. In 2019, there were an estimated 409 000 malaria deaths globally. Approximately 94 per cent of deaths were in sub-Saharan Africa.
Yet, in recent years, WHO and its partners have reported a “stagnation in progress” in the fight against the deadly disease and called for the development of new tools, including vaccines, to get malaria control efforts back on track.
Now, WHO says the use of RTS,S, in combination with existing prevention measures, will see the pace of malaria progress in Africa increase, improving child health and saving lives.
“Today’s recommendation offers a glimmer of hope for the continent which shoulders the heaviest burden of the disease ….” Dr Matshidiso Moeti, WHO Regional Director, Africa.
“For centuries, malaria has stalked sub-Saharan Africa, causing immense personal suffering,” said Dr Matshidiso Moeti, WHO Regional Director for Africa.
“We have long hoped for an effective malaria vaccine and now for the first time ever, we have such a vaccine recommended for widespread use. Today’s recommendation offers a glimmer of hope for the continent which shoulders the heaviest burden of the disease and we expect many more African children to be protected from malaria and grow into healthy adults.”
WHO has already completed a successful pilot program of three doses of the vaccine in three African countries – Ghana, Kenya and Malawi – reaching more than 800,000 children since 2019.
RTS,S resulted in a significant reduction in life-threatening severe malaria and pediatric hospitalisation with malaria infection in the program. It was also found to be highly cost-effective and its delivery to be feasible. And most importantly, the vaccine has been found to have a favourable safety profile in the more than 2.3 million doses of the vaccine administered.

The RTS,S malaria vaccine is best used with existing tools such as insecticide-treated mosquito nets.
However, WHO does not claim that the vaccine is a ‘silver-bullet solution’. While data showed the vaccine to be an effective tool in guarding against Plasmodium falciparum – the deadliest malaria parasite globally and the most prevalent in Africa – it is best used “on top of existing tools” – such as insecticide-treated mosquito nets.
Accordingly, WHO advises countries to move away from a “one-size fits all” approach to fighting malaria and apply a mix of tools – including RTS,S – tailored to local contexts for maximum benefit.
While several malaria vaccines are currently in development, RTS,S is the first malaria vaccine to complete the clinical development process and receive a positive scientific opinion from the European Medicines Agency (EMA), a stringent regulatory authority.
WHO says a second malaria vaccine could be highly beneficial to malaria control, particularly because it could increase supply to meet the anticipated high demand.
RTS,S pilot programs will continue in the three pilot countries of Ghana, Kenya and Malawi through to 2023 to understand the added value of the 4th vaccine dose and measure the longer-term impact on child deaths. In each of these countries, the Ministries of Health have led the pilots through each country’s routine immunisation systems and will continue to do so.
While the pilot program is coordinated by WHO and supported by in-country, there are several international partners involved, including PATH, UNICEF, and GlaxoSmithKline (GSK) – a British multinational pharmaceutical company that is donating up to 10 million doses of the vaccine for the pilot.
Finance for the pilot program was raised through what WHO describes as an “unprecedented collaboration” among three key global health funding bodies: Gavi, the Vaccine Alliance; the Global Fund to Fight AIDS, Tuberculosis and Malaria; and Unitaid.
As for the RTS,S vaccine’s development, WHO says it is “the result of 30 years of research and development by GSK and through a partnership with PATH, with support from a network of African research centres. The Bill & Melinda Gates Foundation provided catalytic funding for late-stage development of RTS,S between 2001 and 2015.”
Email This Story
Why not send this to a friend?